Secure Product
Development Framework (SPDF)
Secure Medical Devices Throughout Their Lifecycle with Our Robust SPDF
Our Secure Product Development Framework (SPDF) helps you identify and reduce the number and severity of vulnerabilities in your products throughout their entire lifecycle. The SPDF integrates seamlessly with your Quality Management System, and contains cybersecurity-specific procedures and templates that uniquely address your medical device cybersecurity requirements – essential since the Federal Food, Drug & Cosmetic Act mandates assurance that devices are cybersecure.
Our SPDF Services & Templates
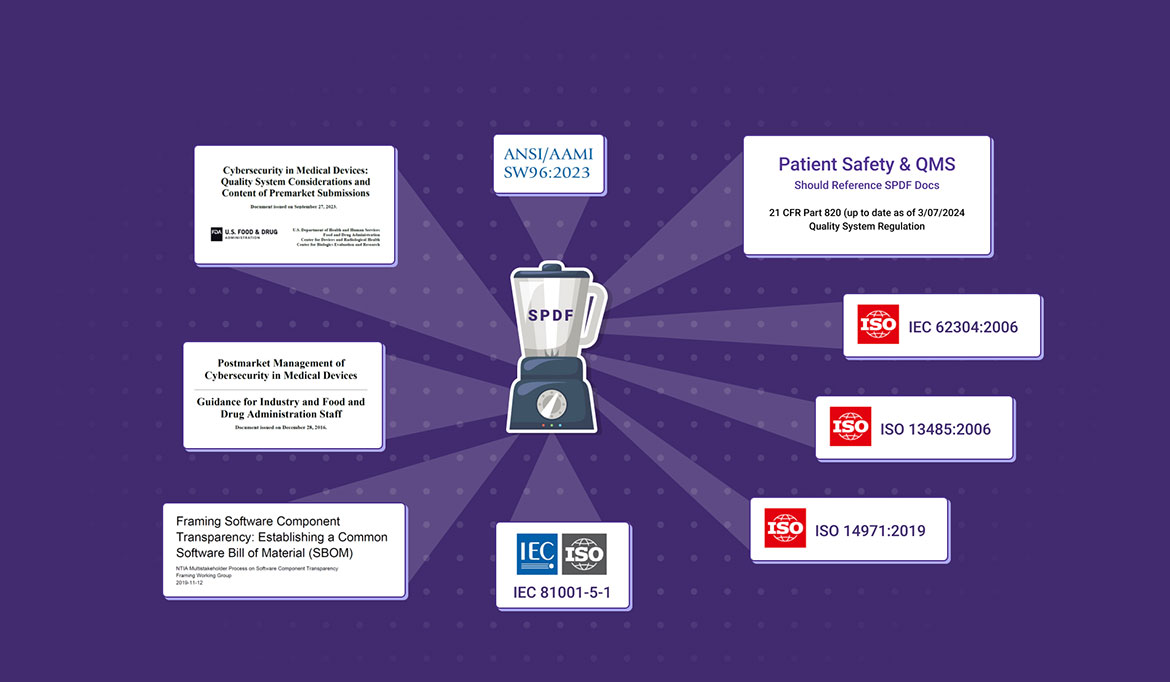
Our services, cybersecurity SPDF procedures, templates and SPDF documentation package are based on standards IEC 81001-5-1 and ANSI/AAMI SW96:2023, which are recognized by the FDA and provide an excellent foundation for a risk-based cybersecurity QMS. With our SPDF, which covers all requirements and recommendations, you can meet quality system regulations and FDA expectations.
Elements Included in Our Cybersecurity SPDF
SPDF Procedures &
Templates
25 procedures with 19 templates that guide SPDF activities for everything needed to successfully navigate an FDA inspection.
SPDF
Manual
A Cybersecurity Management System manual for medical devices.
FDA Premarket
Submission Templates
19 templates covering all required documentation that directly matches eSTAR terminology.
Additional Services
Combined Premarket Submission & SPDF Services
We will generate everything required for your premarket submission, as well as install and train your team on using our SPDF.
SPDF Integration Services
We'll ensure smooth integration between your existing QMS/risk management processes and our SPDF.